Intermittent Hypoxic Training (IHT) involves brief periods of breathing air that’s modified to provide mild controlled doses of hypoxic (oxygen reduced) air for the purpose of producing a range of beneficial physiological adaptations similar to those that can occur when humans adapt to high altitude environments.
Athletes make use of various high altitude training and living environments to improve strength, speed and other aspects of performance (Gough, Saunders et al 2012). Over 80% of Olympic medal winners have made use of altitude training and both the Australian and NSW institutes of sport have altitude training chambers.
Non-athletes, including the elderly have also been shown to benefit with improvements in various markers of aerobic fitness, circulation and breathing (Burtscher, Pachinger et al 2004). General health can also improve in non-athletes who spend time at altitude. The broad improvements in body function that have been reported from moderate and prolonged stays at altitude suggest better immune function, increased anti-oxidant production,enhanced metabolic function, improved glycemic control as well as better blood flow and breathing (Singh 1977, Larsen, Hansen et al. 1997, Lee, Chen et al. 2003). Some conditions found to improve in high altitude environments include asthma, diabetes, depression, anxiety, gastrointestinal disease and obesity (Singh 1977, Kayser and Verges 2013, Kong, Zang et al. 2014, Wang, Liu et al. 2014).
Decades of research in the former Soviet Union showed that IHT could achieve the same benefits as sustained exposure to high altitude conditions (Serebrovskaya 2002).
What’s involved?
During IHT the person alternates breathing oxygen reduced air with room air or with oxygen enriched air, through intervals typically several minutes long. IHT breathing programs are individually selected for each individual in accordance with their adaptive capacity, condition and training objectives.
A sample IHT program can involve 10-30 sessions which involve breathing moderate hypoxia (9–16% inspired O2) for 3-7 minutes alternating with normoxia or hyperoxia (21-35%O2) for about 3-5 minutes for 60 minutes.
Hypoxic conditioning – Tool of adaptive medicine
Oxygen is the most essential element for our survival, and the adverse consequences of hypoxia are well known, so its surprising to many to hear that controlled exposure to oxygen deficit (or hypoxia) if within a person’s adaptive range, can actually promote longevity, enhance physical performance and even help to create conditions that support healing in certain diseases (Serebrovskaya 2002, Verges, Chacaroun et al. 2015).
The reason this occurs is that brief, intermittent exposure to hypoxia stimulates a range of the body’s adaptive responses through epigenetic changes and increased expression of genes like hypoxia inducible factor (HIF), uncoupling protein 2 (UCP2) and methyl-tetrahydrofolate reductase (MTHFR) that are involved in angiogenesis, glycolysis, lactate shuttling, glucose transport, capillary density and mitochondrial function. Hypoxia has protective effects on the cell and on DNA because it induces p53, a transcription regulation factor that is sometimes called the “guardian of the genome”. It has a protective and repairing role in DNA damage.
The end result of hypoxia adaptation is improved oxygen, better circulation, improved mitochondrial function, increased tolerance to various stressors and even toxic chemicals, increased antioxidant production and reduced inflammation (Kayser and Verges 2013, Verges, Chacaroun et al. 2015). These adaptive changes enhance physical and mental capacity, so that the body is better able to cope with a range of stressors and repair and heal cells tissues and organs. The Russians have led the research in this field since the early 1960’s and have been applying what they have found to medicine, sport and physical conditions (Serebrovskaya 2002). In recent years research done in the USA, Europe and China has added to the body of knowledge on IHT and its clinical applications.
Cross adaptation
Other far reaching effects on general function of the organism occur because of the principle of “cross adaptation’ (Meerson 1993). Adaptation to one type of stress or load will, to some extent, increase the body’s ability to cope with stresses of another type. It is well known that a regular exercise program is associated with an increased tolerant to stress. Stress-related diseases such as hypertension, heart disease, ulceration of the stomach or duodenum, diabetes, dermatological diseases and disordered immunity have all been shown to improve with both exercise and IHT. Protection comes because the body becomes more tolerant and resistant to stress. Dr. F.Z. Meerson, a key researcher in the field of Adaptive Medicine, describes how a stressor can increase stress resilience:
- By inducing a fade away of the stress reaction
- By increasing activity of the central and peripheral stress limiting systems
- By desensitization of target organs.
Other key physiological mechanisms of IHT
Oxygen and blood flow- Hypoxia leads to the production of hypoxia-inducible-factor (HIF-1). This leads to production of vascular endothelial growth factor (VEGF) and stimulates EPO production.
Improvement in the blood oxygen transport capacity induces altitude acclimatisation (Rodriguez FA, Casas H et al. 1999, Casas, Casas et al. 2000, Costa, Alva et al. 2013). It is also associated with increased anaerobic threshold and blood lactate kinetics (Rodriguez FA, Casas H et al. 1999, Casas, Casas et al. 2000, Koistinen, Rusko et al. 2000, Lippi, Franchini et al. 2011)
Nitric Oxide- IHT increases endothelial production of nitric oxide (NO) which has the short term effect of increasing regional cerebral blood flow and augmenting the vaso- and neuroprotective effects of endothelial NO in conditions such a hypertension. However, the body also needs to be protected from excessive production of NO by microglyia, astrocytes and cortical neurons as this generates neurotoxic peroxynitrite. Controlled exposure to IHT which promotes adaptation to hypoxia prevents NO overproduction in the brain and other tissues, and enhances storage of excessive NO in the form of S-nitrosothiols and dinitrosyl iron complexes. The end result is neuro-protection of various pathologies, reduced blood pressure (Lyamina, Lyamina et al. 2011) and a reduction of oxidative stress (Malyshev, Bakhtinia et al. 2001, Manukhina, Downey et al. 2016).
Hypoxia and Stem Cells- Stem Cells mostly arise from our bone marrow and are involved in regenerating and repairing body tissues damaged from daily wear and tear. Stem cells are most abundant in fetal circulation where oxygen levels are very low. They disappear from circulation soon after birth but survive in various hypoxic niches in adulthood. They act as a pool of undifferentiated cells that can be used to repair bone, cartilage, muscle, blood vessels, nerves and other body tissues. They can undergo trans-differentiation to other cell types, such as liver cells, neurons, cardiac myocytes and vascular endothelium.
The quantity of stem cells in peripheral blood fluctuates and various stressors including exercise, inflammation and hypoxia can mobilise stem cells into circulation so that they can then move to areas where they do their regenerative work.
Stem cells are also important in immune function, helping to prevent infections and tissue damage. Intermittent hypoxic training has been found to mobilise stem cells and improve immunity in adult men. A 2-week program of 4 cycles of cyclic, 5-min exposures to hypoxia altered stem cell migration and increased circulating platelets, and augmented phagocytic and bactericidal activities of neutrophils, while suppressing pro-inflammatory cytokines (Serebrovskaya, Nikolsky et al. 2011) .
Disease conditions where the stimulating effects of intermittent hypoxia on stem cells can improve health include type 2 diabetes mellitus, coronary artery disease, osteoarthritis, Parkinson’s disease and chronic renal failure (Malshe 2011).
Hypoxia – a matter of dose
Intermittent hypoxia (IH) has been a subject of considerable investigation from the viewpoint of its adverse and beneficial effects. Recent studies reveal that IH has varied effects on multiple body systems and that the magnitude of these effects (and even the direction) depends on the IH “dose”. With low cycles-per-day counts and/or mild to moderate hypoxic episodes beneficial effects are predominant (Navarrete-Opazo and Mitchell 2014, Verges, Chacaroun et al. 2015). Low doses of IH in the body’s adaptive range can have a directly opposite effect to those demonstrated in patients with sleep apnea, respiratory insufficiency, stroke or infarct (Serebrovskaya, Manukhina et al. 2008, Burtscher, Haider et al. 2009). Notably there have been no reported cases of adverse events from clinical use of IHT in the scientific literature (Rosalba Courtney 2015-unpublished literature review).
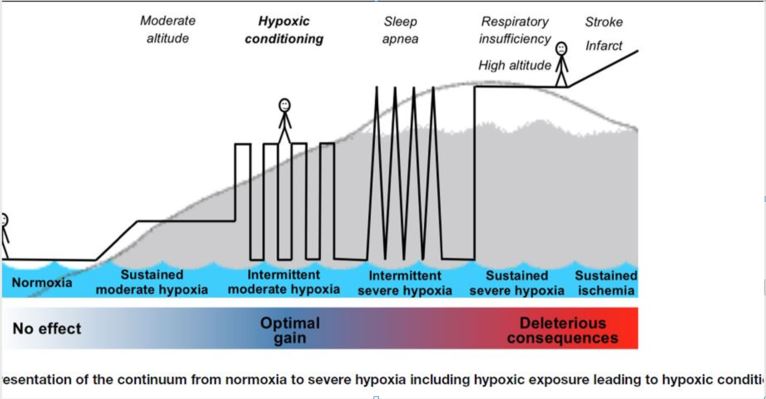
Image from From Verges- 2015 Frontiers in Pediatrics- “Hypoxic Conditioning as a New Therapeutic Modality”.(Verges, Chacaroun et al. 2015)
Literature on specific conditions and IHT
(English language research and reviews only)
| Respiratory – Improve COPD/bronchial asthma clinical symptoms without unwanted side effects.Review showing treatment of asthma and COPD to be one of the most studied applications of IHT, Over 20 years of clinical usage and research in Russia and Ukraine showing no adverse effects and consistent improvement. | (Serebrovskaya, Swanson et al. 2003) |
| Respiratory – Beneficial effects in mild COPD patients, including increased exercise tolerance, improved autonomic nervous system function, improved lung function (hypercapnic ventilatory response, forced expiratory volume in 1s, and forced vital capacity). | (Haider, Casucci et al. 2009, Burtscher, Gatterer et al. 2010) |
| Respiratory – Improve respiratory (and nonrespiratory) motor output after spinal cord injury and in amyotropic lateral sclerosis (ALS). | (Mitchell 2007) |
| Immune – Enhance the innate immune system (e.g. increase neutrophils’ phagocytic and bactericidal activities) while having an overall anti-inflammatory effect (suppressing pro-inflammatory mediators by more than 90%) in healthy humans. | (Serebrovskaya TV, Nikolsky IS et al. 2011) |
| Cardiovascular – Decrease systolic pressure by up to 30 mmHg and diastolic pressure by up to 15 mmHg in in individuals with hypertension. | (Serebrovskaya, Manukhina et al. 2008) |
| Cardiovascular – Increased nitric oxide synthesis and decreased BP to values similar to those of normotensive individuals. Blood pressure reduction persisted for at least 3 months in 28 of 33 hypertensive subjects. | (Lyamina, Lyamina et al. 2011) |
| Cardiovascular – Protective against myocardial injuries and attenuate against subsequent ischemic incidence. Review by Zhuang discusses variety of protective effects including neurohumoral alterations. Clinical protocols of IHT can avoid adverse effects caused by high dose IH and chronic continuous hypoxia. | (Zhuang J and Z. 1999, Dirnagl, Becker et al. 2009) |
| Neuromuscular – Therapeutic benefits following cervical spinal injury and improved motor output. | (Mitchell 2007, Tester, Fuller et al. 2014) |
| Neuromuscular – Improvement in limb function in motor incomplete, chronic (<1 year) SCI patients (American SCI Association Impairment Scale C or D). | (Trumbower, Jayaraman et al. 2012) |
| Neuromuscular – Increase walking speed (10-m walk test) and distance in SCI patients by up to 18% 3 days after treatment. | (Hayes, Jayaraman et al. 2014) |
| Brain and Nervous System – Hypoxic preconditioning at adapative dose can stimulates neurogenesis, it increases resistance to effect of toxic chemical substances and is protective against cerebral ischemia from various causes (Basovich 2013). | (Basovich 2013) |
| Brain and Nervous System – Protect cerebrovascular function in Alzheimers disease, an important modifiable risk factor for Alzheimer’s disease. Treatment of experimental models of AD showed that intermittent hypoxic training (IHT) improved the health of both cerebral and extra-cerebral blood vessels, prevented the loss of neurons in the cortex and improved memory. Beneficial effects of IHT has also been shown for other neuropathologies including dyscirculatory encephalopathy, ischemic stroke injury, audiogenic epilepsy, spinal cord injury, and alcohol withdrawal. | (Manukhina, Downey et al. 2016) |
| Brain and Nervous System – Attenuate the response to subsequent ischemic incidents, preconditioning the brain and promoting endogenous mechanisms for recovery of noxious stimuli and recovery from damage. | (Dirnagl, Becker et al. 2009) |
| Brain and Nervous System – No detrimental changes after IHT in spasticity, heart rate or cognitive function, in spinal cord injury patients following IHT. | (Hayes, Jayaraman et al. 2014) |
| Brain and Nervous System – Enhance exercise’s positive effects on sleep quality & cognitive function in the elderly. | (Schega, Peter et al. 2013) |
| Brain and Nervous System – No detrimental effect after IHT on alertness, vigilance, or working memory in young healthy adults. | (Rodriguez FA, Casas H, Casas M, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J and V. G. 1999) |
| Brain and Nervous System – No detrimental effect on measures of cognitive function. | (Ando S, Hatamoto Y et al. 2013, Schega, Peter et al. 2013) |
| Metabolism – Beneficial effects on metabolism, including reduced body weight, cholesterol, and blood sugar levels, as well as increased insulin sensitivity. | (Netzer, Chytra et al. 2008, Mackenzie, Maxwell et al. 2011, Urdampilleta, Gonzalez-Muniesa et al. 2012, Fuller and Courtney 2016) |
| Metabolism – Improvements in metabolic markers are accompanied by improved cardiorespiratory function and improved exercise capacity. | (Shi, Watanabe et al. 2013) |
| Metabolism – A beneficial effect of moderate controlled hypoxia has been shown for a number of metabolic parameters associated with obesity including improved insulin sensitivity and glucose tolerance, and decreased cholesterol levels (Haufe, Wiesner et al. 2008) [6,7]. | (Haufe, Wiesner et al. 2008) |
| Sleep Apnoea – Improved neuroplasticity of breathing control that in presence of normal CO2 mitigates sleep apnoea. | (Mateika and Syed 2013) |
| Sleep Apnoea – While obstructive sleep apnoea often leads to persistent, maladaptive chemoreflex-mediated activation of the sympathetic nervous system which culminates in hypertension, controlled IHT conditioning programs can be safe, efficacious modalities for prevention and treatment of hypertension. This article reviews outcomes of Soviet research and describes research on mechanisms. | (Serebrovskaya, Manukhina et al. 2008) |
| Oxygen – Improved blood oxygen transport capacity. | (Rodriguez FA, Casas H et al. 1999, Casas, Casas et al. 2000, Koistinen, Rusko et al. 2000, Lippi, Franchini et al. 2011) |
| Oxygen – Induced altitude acclimatisation. | (Casas, Casas et al. 2000, Billaut, Gore et al. 2012, Costa, Alva et al. 2013) |
| Physical Performance – Increase aerobic capacity/endurance/athletic performance. | (Rodriguez FA, Casas H et al. 1999, Casas, Casas et al. 2000, Costa, Alva et al. 2013) |
| Physical Performance – Induce a range of physiological adaptations that enhance fitness and athletic performance. | (Burtscher, Pachinger O et al. 2004)
(Bonetti and Hopkins 2009, Lippi, Franchini et al. 2011)(Bazzani 2007) |
| Physical Performance – Improved energy efficiency, exercise capacity and various measures of performance. | (Czuba, Waskiewicz et al. 2011, Czuba, Zając et al. 2013, Czuba, Maszczyk et al. 2014) |
| Physical Performance – Age related – Fitness improvements can be greater in untrained older individuals with greater positive effects on hemodynamics, microvascular endothelial function, and work capacity. | (Shatilo, Korkushko et al. 2008).
|
| Physical Performance – Age related – Increase peak oxygen consumption and diminish heart rate, systolic blood pressure, blood lactate concentration and perceived exertion during submaximal exercise in 50–70 YO men. Changes in responses to exercise after IH were similar in subjects with and without prior myocardial infarction. | (Burtscher, Pachinger O et al. 2004).
|
For an assessment or to book an IHT course contact Rosalba here
REFERENCES
Ando S, Hatamoto Y, Sudo M, Kiyonaga A, Tanaka H and H. Y (2013). “The effects of exercise under hypoxia on cognitive function.” PLoS One 8e63630, .
Basovich, S. N. (2013). “Trends in the use of preconditioning to hypoxia for early prevention of future life diseases.” Biosci Trends7(1): 23-32.
Bazzani, C., Ed. (2007). Enhancing Therapeutic Compliance. Fundamentals of Sleep Technology, Lippincott Williams & Wilkins.
Billaut, F., C. J. Gore and R. J. Aughey (2012). “Enhancing Team-Sport Athlete Performance.” Sports Medicine42(9): 751-767.
Bonetti, D. L. and W. G. Hopkins (2009). “Sea-Level Exercise Performance Following Adaptation to Hypoxia: A Meta-Analysis.” Sports Medicine39(2): 107-127.
Burtscher, M., H. Gatterer, C. Szubski, E. Pierantozzi and M. Faulhaber (2010). “Effects of interval hypoxia on exercise tolerance: special focus on patients with CAD or COPD.” Sleep and Breathing14(3): 209-220.
Burtscher, M., T. Haider, W. Domej, T. Linser, H. Gatterer, M. Faulhaber, E. Pocecco, I. Ehrenburg, E. Tkatchuk, R. Koch and L. Bernardi (2009). “Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD.” Respir Physiol Neurobiol165(1): 97-103.
Burtscher, M., Pachinger O, I. Ehrenbourg, G. Mitterbauer, M. Faulhaber, R. Puhringer and E. Tkatchouk (2004). “Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease.” Int J Cardiol 96: 247-254.
Casas, M., H. Casas, T. Pages, R. Rama, A. Ricart, J. L. Ventura, J. Ibanez, F. A. Rodriguez and G. Viscor (2000). “Intermittent hypobaric hypoxia induces altitude acclimation and improves the lactate threshold.” Aviat Space Environ Med71: 125-130.
Costa, D. C., N. Alva, L. Trigueros, A. Gamez, T. Carbonell and R. Rama (2013). “Intermittent Hypobaric Hypoxia Induces Neuroprotection in Kainate-Induced Oxidative Stress in Rats.” Journal of Molecular Neuroscience50(3): 402-410.
Czuba, M., A. Maszczyk, D. Gerasimuk, R. Roczniok, O. Fidos-Czuba, A. Zajac, A. Golas, A. Mostowik and J. Langfort (2014). “The effects of hypobaric hypoxia on erythropoiesis, maximal oxygen uptake and energy cost of exercise under normoxia in elite biathletes.” J Sports Sci Med13(4): 912-920.
Czuba, M., Z. Waskiewicz, A. Zajac, S. Poprzecki, J. Cholewa and R. Roczniok (2011). “The effects of intermittent hypoxic training on aerobic capacity and endurance performance in cyclists.” Journal Of Sports Science & Medicine10(1): 175-183.
Czuba, M., A. Zając, A. Maszczyk, R. Roczniok, S. Poprzecki, W. Garbaciak and T. Zając (2013). “The Effects of High Intensity Interval Training in Normobaric Hypoxia on Aerobic Capacity in Basketball Players.” Journal of Human Kinetics39: 103-114.
Dirnagl, U., K. Becker and A. Meisel (2009). “Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. .” Lancet Neurol8: 398-412.
Fuller, N. R. and R. Courtney (2016). “A case of remission from pre-diabetes following intermittent hypoxic training.” Obesity Research & Clinical PracticeIn Press.
Gough, C. E., P. U. Saunders, J. Fowlie, B. Savage, D. B. Pyne, J. M. Anson, N. Wachsmuth, N. Prommer and C. J. Gore (2012). “Influence of altitude training modality on performance and total haemoglobin mass in elite swimmers.” European Journal of Applied Physiology112(9): 3275-3285.
Haider, T., G. Casucci, T. Linser, M. Faulhaber, H. Gatterer, G. Ott, A. Linser, I. Ehrenbourg, E. Tkatchouk, M. Burtscher and L. Bernardi (2009). “Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease.” J Hypertens27(8): 1648-1654.
Haufe, S., S. Wiesner, S. Engeli, F. C. Luft and J. Jordan (2008). “Influences of normobaric hypoxia training on metabolic risk markers in human subjects.” Med Sci Sports Exerc40(11): 1939-1944.
Hayes, H. B., A. Jayaraman, M. Herrmann, G. S. Mitchell, W. Z. Rymer and R. D. Trumbower (2014). “Daily intermittent hypoxia enhances walking after chronic spinal cord injury.” Neurology82: 104-113.
Kayser, B. and S. Verges (2013). “Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies.” Obes Rev14(7): 579-592.
Koistinen, P. O., H. Rusko, K. Irjala, A. Rajamaki, K. Penttinen, V. Sarparanta, J. Karpakka and J. Leppaluoto (2000). “EPO, red cells, and serum transferrin receptor in continuous and intermittent hypoxia. .” Med Sci Sports Exerc 32: 800-804.
Kong, Z., Y. Zang and Y. Hu (2014). “Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults.” Sleep Breath18(3): 591-597.
Larsen, J. J., J. M. Hansen, N. V. Olsen, H. Galbo and F. Dela (1997). “The effect of altitude hypoxia on glucose homeostasis in men.” J Physiol504 ( Pt 1): 241-249.
Lee, W. C., J. J. Chen, H. Y. Ho, C. W. Hou, M. P. Liang, Y. W. Shen and C. H. Kuo (2003). “Short-term altitude mountain living improves glycemic control.” High Alt Med Biol4(1): 81-91.
Lippi, G., M. Franchini and G. Banfi (2011). “Normobaric hypoxia and sports: the debate continues.” European Journal of Applied Physiology111(1): 159-160.
Lyamina, N. P., S. V. Lyamina, V. N. Senchiknin , R. T. Mallet, H. F. Downey and E. B. Manukhina (2011). “Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. .” J Hypertens 2929: 2265-2272.
Mackenzie, R., N. Maxwell, P. Castle, G. Brickley and P. Watt (2011). “Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes.” Diabetes Metab Res Rev27(1): 94-101.
Malshe, P. C. (2011). “Nisshesha rechaka pranayama offers benefits through brief intermittent hypoxia.” Ayu32(4): 451-457.
Malyshev, I. Y., L. Y. Bakhtinia, T. A. Zenina, V. D. Mikoyan, L. N. Kubrina, A. F. Vanin and E. B. Manukhina (2001). “Nitric Oxide (NO) dependant mechanisms of adaptation to hypoxia.” Hypoxia Medical Journal3(1): 23.
Manukhina, E. B., H. F. Downey, X. Shi and R. T. Mallet (2016). “Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease.” Exp Biol Med (Maywood)241(12): 1351-1363.
Mateika, J. H. and Z. Syed (2013). “Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations.” Respir Physiol Neurobiol188(3): 289-300.
Meerson, F. (1993). Essentials of adaptive medicine: Protective effects of adaptation. Geneva, Hypoxia Medical.
Mitchell, A. (2007). Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. Genetic Basis for Respiratory Control Disorders. C. Gaultier. New York, Springer: 291-311.
Navarrete-Opazo, A. and G. S. Mitchell (2014). “Therapeutic potential of intermittent hypoxia: a matter of dose.” Am J Physiol Regul Integr Comp Physiol307(10): R1181-1197.
Netzer, N. C., R. Chytra and T. Kupper (2008). “Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia.” Sleep Breath12(2): 129-134.
Rodriguez FA, Casas H, Casas M, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J and V. G. (1999). “Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity.” Med Sci Sports Exerc 31: 264-268.
Schega, L., B. Peter, A. Törpel, H. Mutschler, B. Isermann and D. Hamacher (2013). “Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study.” Gerontology59(4): 316-323.
Serebrovskaya, T. V. (2002). “Intermittent hypoxia research in the former Soviet Union and the Commonwealth of Independent States: History and review of the concept and selected applications.” High Altitude Medicine and Biology3(2): 205-221.
Serebrovskaya, T. V. (2002). “Intermittent hypoxia research in the former soviet union and the commonwealth of independent States: history and review of the concept and selected applications.” High Altitude Medicine & Biology3(2): 205-221.
Serebrovskaya TV, Nikolsky IS, Nikolska VV, Mallet RT and I. VA. (2011). “Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men.” High Alt Med Biol 12: 243-252.
Serebrovskaya, T. V., B. Manukhina, M. L. Smith, H. Downey and R. T. Mallet (2008). “Intermittent hypoxia: cause of or therapy for systemic hypertension? .” Exp Biol Med Maywood 233: 627-650.
Serebrovskaya, T. V., I. S. Nikolsky, V. V. Nikolska, R. T. Mallet and V. A. Ishchuk (2011). “Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men.” High altitude medicine & biology12(3): 243-252.
Serebrovskaya, T. V., R. J. Swanson and E. E. Kolesnikova (2003). “Intermittent hypoxia: mechanisms of action and some applications to bronchial asthma treatment.” Journal Of Physiology And Pharmacology: An Official Journal Of The Polish Physiological Society54 Suppl 1: 35-41.
Shatilo, V. B., O. V. Korkushko, V. A. Ischuk, H. F. Downey and T. V. Serebrovskaya (2008). “Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men.” High Alt Med Biol 9: 43-52.
Shi, B., T. Watanabe, S. Shin, T. Yabumoto and T. Matsuoka (2013). “Effect of normobaric hypoxia on cardiorespiratory and metabolic risk markers in healthy subjects.” Advances in Bioscience and Biotechnology4(3): 340-345.
Singh, I., Chohan, I.S., Lal, M., Khanna, P.K., Srivastava, M.C., Nanda, R.B., Lamba, J.S., Malhotra, M.S. (1977). “Effects of high altitude stay on the incidence of common diseases in man.” Int. J. Biometeor21(2): 93-122.
Tester, N. J., D. D. Fuller, J. S. Fromm, M. R. Spiess, A. L. Behrman and J. H. Mateika (2014). “Long-term facilitation of ventilation in humans with chronic spinal cord injury.” Am J Respir Crit Care Med 189: 57-65.
Trumbower, R. D., A. Jayaraman, G. S. Mitchell and W. Z. Rymer (2012). “Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury.” Neurorehabil Neural Repair 26: 163-172.
Urdampilleta, A., P. Gonzalez-Muniesa, M. P. Portillo and J. A. Martinez (2012). “Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity.” J Physiol Biochem68(2): 289-304.
Verges, S., S. Chacaroun, D. Godin-Ribuot and S. Baillieul (2015). “Hypoxic Conditioning as a New Therapeutic Modality.” Front Pediatr3: 58.
Wang, R., D. Liu, X. Wang, W. Xiao, N. Wu, B. Gao and P. Chen (2014). “The effect of ‘sleep high and train low’ on weight loss in overweight Chinese adolescents: study protocol for a randomized controlled trial.” Trials15: 250.
Zhuang J and Z. Z. (1999). “Protective effects of intermittent hypoxic adaptation on myocardium and its mechanisms. .” Biol Signals Recept 8: 316-322.







